Details of experiments between the fragments Q5VZ89~1320-1329 and P61981~1-247
Note that the results of all experiments are listed, regardless of the modification states of the fragments.
Experiment series 1
Protein A protein: DENND4C
Protein A fragment: 1320-1329
Protein A site: 14-3-3_pSer1325
Protein A modification: (SEP)1325
Protein A sequence: KERSTSLSAL
Protein A construct: KERST(pSer)LSAL (crude synthetic peptide)
Protein B protein: YWHAG
Protein B fragment: 1-247
Protein B site: 14-3-3
Protein B modification: 100_uM_FCA
Protein B construct: avi-his6-MBP-TEVsite-14-3-3gamma
Average holdup BI: 0.81
Holdup BI standard deviation: 0.01
Immobilized partner concentration in holdup experiment (10-6M): 115
P value (usually -log10(P Two-sided unpaired T-test), but double check the experimental details): 8.88
Experiment method: HU Multiplex
Details of affinity fitting: hyperbolic binding equation
Date of measurement: 2022.02.17.
Experimental details: -log10(P Two-sided unpaired T-test (4 vs 4))
Number of measurements: 4
Measured pKd: 4.58
Experiment series 2
Protein A protein: DENND4C
Protein A fragment: 1320-1329
Protein A site: 14-3-3_pSer1325
Protein A modification: (SEP)1325
Protein A sequence: KERSTSLSAL
Protein A construct: KERST(pSer)LSAL (crude synthetic peptide)
Protein B protein: YWHAG
Protein B fragment: 1-247
Protein B site: 14-3-3
Protein B construct: avi-his6-MBP-TEVsite-14-3-3gamma
Average holdup BI: 0.87
Holdup BI standard deviation: 0.02
Immobilized partner concentration in holdup experiment (10-6M): 129
P value (usually -log10(P Two-sided unpaired T-test), but double check the experimental details): 7.84
Experiment method: HU Multiplex
Details of affinity fitting: hyperbolic binding equation
Date of measurement: 2021.10.13.
Experimental details: -log10(P Two-sided unpaired T-test (4 vs 5))
Number of measurements: 5
Measured pKd: 4.7
Experiment series 3
Protein A protein: DENND4C
Protein A fragment: 1320-1329
Protein A site: 14-3-3_pSer1325
Protein A modification: (SEP)1325
Protein A sequence: KERSTSLSAL
Protein A construct: KERST(pSer)LSAL (crude synthetic peptide)
Protein B protein: YWHAG
Protein B fragment: 1-247
Protein B site: 14-3-3
Protein B construct: avi-his6-MBP-TEVsite-14-3-3gamma
Average holdup BI: 0.85
Holdup BI standard deviation: 0.01
Immobilized partner concentration in holdup experiment (10-6M): 134
P value (usually -log10(P Two-sided unpaired T-test), but double check the experimental details): 7.74
Experiment method: HU Multiplex
Details of affinity fitting: hyperbolic binding equation
Date of measurement: 2022.02.17.
Experimental details: -log10(P Two-sided unpaired T-test (4 vs 4))
Number of measurements: 4
Measured pKd: 4.63
Experiment series 4
Protein A protein: DENND4C
Protein A fragment: 1320-1329
Protein A site: 14-3-3_pSer1325
Protein A modification: (SEP)1325
Protein A sequence: KERSTSLSAL
Protein A construct: biotin-ttds-KERST(pSer)LSAL (HPLC-purified synthetic peptide)
Protein B protein: YWHAG
Protein B fragment: 1-247
Protein B site: 14-3-3
Protein B construct: avi-his6-MBP-TEVsite-14-3-3gamma
Measured dissociation constant (10-6M): 24
Standard deviation of dissociation constant (10-6M): 2.5
Experiment method: competitive Fluorescence Polarization
Details of affinity fitting: Competitive binding equation in ProFit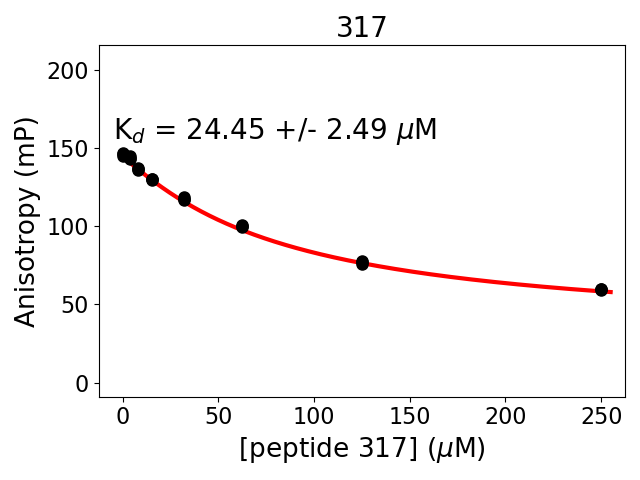
Date of measurement: 2022.01.18
Number of measurements: 3
Measured pKd: 4.61
Experiment series 5
Protein A protein: DENND4C
Protein A fragment: 1320-1329
Protein A site: 14-3-3_pSer1325
Protein A modification: (SEP)1325
Protein A sequence: KERSTSLSAL
Protein A construct: biotin-ttds-KERST(pSer)LSAL (HPLC-purified synthetic peptide)
Protein B protein: YWHAG
Protein B fragment: 1-247
Protein B site: 14-3-3
Protein B construct: avi-his6-MBP-TEVsite-14-3-3gamma
Measured dissociation constant (10-6M): 23
Standard deviation of dissociation constant (10-6M): 2
Experiment method: competitive Fluorescence Polarization
Details of affinity fitting: Competitive binding equation in ProFit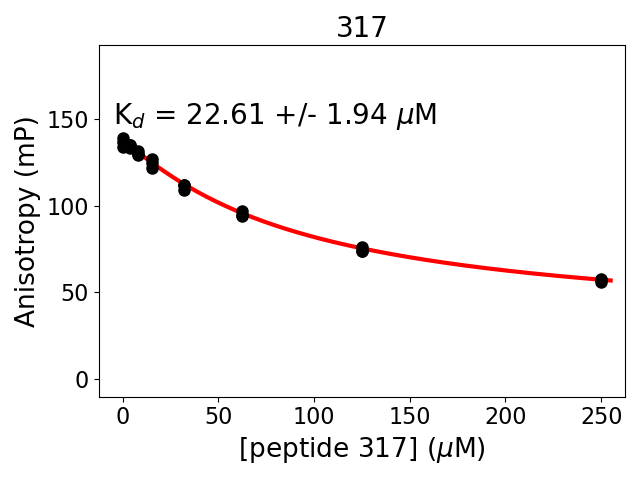
Date of measurement: 2022.01.10
Number of measurements: 3
Measured pKd: 4.65