Details of experiments between the fragments P03126~149-158 and Q96L92~40-141
Note that the results of all experiments are listed, regardless of the modification states of the fragments.
Experiment series 1
Protein A protein: HPV16-E6
Protein A fragment: 149-158
Protein A site: PBM
Protein A modification: (TPO)156
Protein A sequence: SSRTRRETQL
Protein A construct: Biotin-ttds-SSRTRREpTQL
Protein B protein: SNX27
Protein B fragment: 40-141
Protein B site: PDZ
Protein B construct: his6-MBP-TEVsite-PDZ
Average holdup BI: -0.02
Immobilized partner concentration in holdup experiment (10-6M): 18
Experiment method: CALIP_holdup
Details of affinity fitting: BSA and lysozyme internal standards + bacterial cell lysate + normal layout
PUBMED ID of publication: 36115835
Number of measurements: 1
The affinity was below the detection threshold of the assay.
Experiment series 2
Protein A protein: HPV16-E6
Protein A fragment: 149-158
Protein A site: PBM
Protein A sequence: SSRTRRETQL
Protein A construct: biotin-ttds-SSRTRRETQL
Protein B protein: SNX27
Protein B fragment: 40-141
Protein B site: PDZ
Protein B construct: his6-MBP-TEVsite-PDZ
Average holdup BI: 0.51
Holdup BI standard deviation: 0.14
Immobilized partner concentration in holdup experiment (10-6M): 15
Experiment method: CALIP_holdup
Details of affinity fitting: BSA and lysozyme internal standards + bacterial cell lysate + normal layout
PUBMED ID of publication: 36115835
Number of measurements: 8
Measured pKd: 4.91
Experiment series 3
Protein A protein: HPV16-E6
Protein A fragment: 149-158
Protein A site: PBM
Protein A sequence: SSRTRRETQL
Protein A construct: biotin-ttds-SSRTRRETQL
Protein B protein: SNX27
Protein B fragment: 40-141
Protein B site: PDZ
Protein B construct: his6-MBP-TEVsite-PDZ
Measured dissociation constant (10-6M): 17.59
Standard deviation of dissociation constant (10-6M): 1.15
Experiment method: competitive FP
Details of affinity fitting: Competitive binding equation in ProFit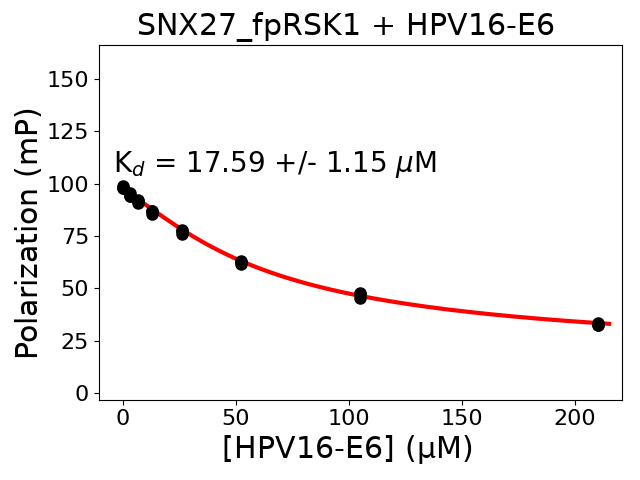
PUBMED ID of publication: 36115835
Number of measurements: 3
Measured pKd: 4.75
Experiment series 4
Protein A protein: HPV16-E6
Protein A fragment: 149-158
Protein A site: PBM
Protein A modification: (TPO)156
Protein A sequence: SSRTRRETQL
Protein A construct: Biotin-ttds-SSRTRREpTQL
Protein B protein: SNX27
Protein B fragment: 40-141
Protein B site: PDZ
Protein B construct: his6-MBP-TEVsite-PDZ
Average holdup BI: 0.02
Normalized holdup BI: 0.02
Immobilized partner concentration in holdup experiment (10-6M): 18
Experiment method: DAPF_holdup
Details of affinity fitting: fluorescein + mCherry internal standards + normalization based on CALIP_holdup data + reverse layout
PUBMED ID of publication: 36115835
Number of measurements: 1
The affinity was below the detection threshold of the assay.
Experiment series 5
Protein A protein: HPV16-E6
Protein A fragment: 149-158
Protein A site: PBM
Protein A sequence: SSRTRRETQL
Protein A construct: biotin-ttds-SSRTRRETQL
Protein B protein: SNX27
Protein B fragment: 40-141
Protein B site: PDZ
Protein B construct: his6-MBP-TEVsite-PDZ
Average holdup BI: 0.56
Holdup BI standard deviation: 0.04
Normalized holdup BI: 0.56
Normalized holdup BI standard deviation: 0.04
Immobilized partner concentration in holdup experiment (10-6M): 18
Experiment method: DAPF_holdup
Details of affinity fitting: fluorescein + mCherry internal standards + normalization based on CALIP_holdup data + reverse layout
PUBMED ID of publication: 36115835
Number of measurements: 6
Measured pKd: 4.91
Experiment series 6
Protein A protein: HPV16-E6
Protein A fragment: 149-158
Protein A site: PBM
Protein A sequence: SSRTRRETQL
Protein A construct: biotin-ttds-SSRTRRETQL
Protein B protein: SNX27
Protein B fragment: 40-141
Protein B site: PDZ
Protein B construct: his6-MBP-TEVsite-PDZ
Average holdup BI: 0.5
Normalized holdup BI: 0.58
Immobilized partner concentration in holdup experiment (10-6M): 18
Experiment method: DAPF_holdup
Details of affinity fitting: fluorescein + mCherry internal standards + normalization based on CALIP_holdup data + reverse layout
Experimental details: not reported internal standards of holdup layout #5
Number of measurements: 1
Measured pKd: 4.94
Experiment series 7
Protein A protein: HPV16-E6
Protein A fragment: 149-158
Protein A site: PBM
Protein A sequence: SSRTRRETQL
Protein A construct: biotin-ttds-SSRTRRETQL
Protein B protein: SNX27
Protein B fragment: 40-141
Protein B site: PDZ
Protein B construct: his6-MBP-TEVsite-PDZ
Average holdup BI: 0.5
Normalized holdup BI: 0.52
Immobilized partner concentration in holdup experiment (10-6M): 18
Experiment method: DAPF_holdup
Details of affinity fitting: fluorescein + mCherry internal standards + normalization based on CALIP_holdup data + reverse layout
Experimental details: not reported internal standards of holdup layout #5
Number of measurements: 1
Measured pKd: 4.84
Experiment series 8
Protein A protein: HPV16-E6
Protein A fragment: 149-158
Protein A site: PBM
Protein A sequence: SSRTRRETQL
Protein A construct: Biotin-ado-ado-SSRTRRETQL
Protein B protein: SNX27
Protein B fragment: 40-141
Protein B site: PDZ
Protein B construct: his6-MBP-TEVsite-PDZ
Average holdup BI: 0.67
Normalized holdup BI: 0.67
Immobilized partner concentration in holdup experiment (10-6M): 18
Experiment method: DAPF_holdup
Details of affinity fitting: fluorescein + mCherry internal standards + normalization based on CALIP_holdup data + reverse layout
Experimental details: Crude peptide synthesis product. The peptide concentration in the affinity conversion step may differ from reality. Not reported internal standards of holdup layout #6
PUBMED ID of publication: 36115835
Number of measurements: 1
Measured pKd: 5.11
Experiment series 9
Protein A protein: HPV16-E6
Protein A fragment: 149-158
Protein A site: PBM
Protein A sequence: SSRTRRETQL
Protein A construct: Biotin-ttds-SSRTRRETQL
Protein B protein: SNX27
Protein B fragment: 40-141
Protein B site: PDZ
Protein B construct: his6-MBP-TEVsite-PDZ
Measured dissociation constant (10-6M): 0
Experiment method: SPR
PUBMED ID of publication: 30726710
Number of measurements: 1
The affinity was below the detection threshold of the assay.